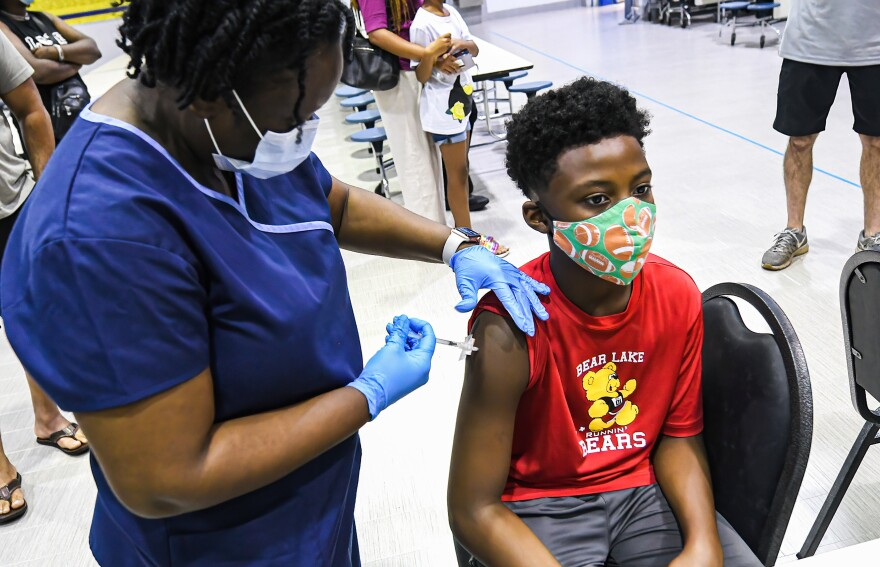
FDA Approves COVID-19 Vaccine For Young Children
November 10, 2021
The FDA recently approved the Pfizer COVID-19 vaccine for children ages 5-11, leaving many parents pleased that their children won’t be at high risk of exposure in school, while others feel that the vaccine will do more harm than good for children of that age. Whether to vaccinate young children has been a controversial topic since vaccines became available, because parents aren’t 100% sure whether the vaccine for children of that age is necessary, as the CDC has stated that young children are at lower risk of contracting the virus.
Though many parents worry about the vaccine and what’s in it, the FDA has taken many precautions to make sure that the vaccine is completely safe yet still effective for children.
“It worried me, but I had family members that had gotten sick with the Coronavirus and it worried me because of how sick they got.” Mrs. Maritza Aguilar, Spanish teacher and parent of a child who would be eligible to receive the vaccine said. “I’m still not sure if I want her to get it because I don’t 100% know what’s in it, but I’m willing to put myself at risk, just not my daughter.”
Different vaccine manufacturers reported that necessary clinical trials involving children as participants are currently in progress. According to the Food and Drug Administration (FDA), “Some [vaccine manufacturers] have stated that they are still enrolling, and some are still administering doses or following participants. This process is expected to include a follow-up period of at least about two months, to allow for proper safety monitoring following the administration of vaccine doses for at least half of the clinical trial vaccine recipients.”
These trials were the first steps taken to safely administer the vaccine to young children.
Once the clinical trials are over, the next step will be to analyze the data from the trials. The vaccine will come in a smaller dose for children ages 5-11, than that for adults. The dosage is 10 micrograms of mRNA, versus the average 30 micrograms given to people ages 12 and above. According to NBC News, “Like in adults, the full vaccination series consists of two doses given three weeks apart. The children’s doses will come in vials with an orange cap and an orange label. The doses for adults and teens come in vials with a purple cap and a purple label.”
According to USA today, the Pfzier vaccine is scheduled to release more data and information regarding the vaccine later this year. Before it officially becomes available to young children it needs to be submitted to the FDA and CDC for approval according to USA today.